INNOVATIVE MEDICAL TECHNOLOGIES
Via Antiniana 2G, 80078 Pozzuoli (NA)
MEDI CORP, PROVIDER OF INNOVATIVE MEDICAL TECHNOLOGY
Medi Corp, the leading supplier of medical technologies in Naples, is committed to promoting cutting-edge medical devices. As a national distributor, we offer technologically advanced solutions to meet the needs of the healthcare industry.
Our mission is to ensure access to high-quality products to improve the health and well-being of patients. With our specialised expertise, we have established ourselves as a reference point in healthcare. We serve as the connection between leading medical companies and healthcare institutions primarily across Campania, Puglia, and the rest of Italy, contributing to innovation and growth in the sector.
Medi Corp's specialised divisions cover a multitude of sectors, including Vascular Surgery, Interventional Cardiology, Cardiothoracic Surgery, General Surgery, and Orthopaedics. We are committed to maintaining our leadership role in the field of medical technologies, providing advanced solutions, and promoting innovation in the healthcare sector. Contact us for further information.
QUALITY MEDICAL DEVICES
Medi Corp is committed to providing its customers in Naples and throughout Italy with high-quality medical devices and professional assistance.
Our products are carefully selected to ensure excellent performance and safety.
Contact us for personalised consultation and discover how we can support your medical practice with tailor-made solutions for your needs.
NATIONAL DISTRIBUTION
PERSONNEL SELECTION
BUSINESS DEVELOPMENT
PUBLIC TENDERS
DRG ANALYSIS AND IDENTIFICATION
CLINICAL TRIALS
LOGISTICS
MARKETING
CONTACT US TO REQUEST INFORMATION
NEWS
Mozec SEB
Mozec SEB by Meril is a next-generation drug-eluting balloon (DEB) with Sirolimus release technology using lipid nanoparticle vectors.
- Unique formulation utilising solid lipid nanoparticles (SLNs) composed of Sirolimus + lipids.
- Sirolimus drug concentration of 3.00 µg/mm².
- Controlled and targeted drug release.
- Diverse-sized nanoparticles enabling drug release at every layer.
- High trackability and pushability for optimal navigability, enhanced by using the Mozec PTCA catheter.
- Low profile tip design and semi-compliant balloon material allowing flat conformity.
- Transparent ergonomic hub displaying balloon dimensions with a flexible distal segment and pushable proximal segment.
SafeGuard Focus
SafeGuard Focus by Merit Medical Systems, Inc. is a mechanical compression device designed for use in patients with pacemakers and implantable cardioverter defibrillators (ICDs).
- Targeted pressure twice that of manual compression devices;
- Improved visibility of the surgical site without device removal;
- Comfortable design with a soft thermoplastic balloon and nylon loop;
- Durable attachment with medical adhesive backing or Velcro® strap;
- Ease of use.
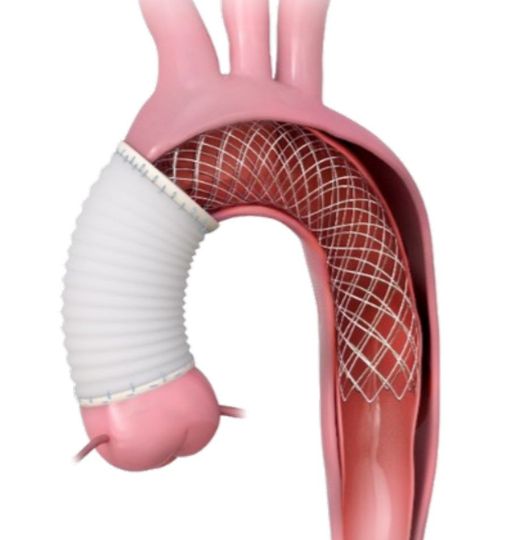
PERSEVERE by Artivion
In 2022, Artivion, Inc. initiated the clinical study PERSEVERE, designed to evaluate the use of the AMDS hybrid prosthesis for the treatment of acute DeBakey type I aortic dissection.
DeBakey type I aortic dissection comprises approximately 50% of dissections and originates from the ascending aorta extending to the aortic arch and sometimes beyond.
PERSEVERE is a prospective, multicentre, non-randomised clinical study consisting of approximately 100 patients in the United States who have undergone acute DeBakey type I aortic dissection.
Each patient will be followed for up to 5 years.
Inclusion criteria include:
- age between 18 and 80 years (male or female) at the time of intervention;
- acute DeBakey type I dissection based on angiography with computed tomography (CTA) and diagnosis ≤14 days from the event;
- presence of malperfusion (cerebral, visceral, renal, spinal cord, and/or peripheral).
Enrolment in the clinical trial began in 2022 and is ongoing, as announced by the company, with the first patient enrolled at the Medical University of South Carolina.